Chemistry for clean environment
(chemical ways to reduce the waste)
Introduction
 The problem with waste. Waste
The problem with waste. Waste or rubbish is what people throw away because they no longer need it or want it. In the real Nature the word ”waste” does not exist – everything thrown away by a creature or naturally dead could be of huge importance for the lifecycle of another creature. In difference, almost every human activity generates waste. The fact that people produce waste, and get rid of it, matters for the following reasons:
- when something is thrown away we lose the natural resources, the energy and the time which have been used to make the product - the majority of resources that we use in manufacturing products and providing services cannot be replaced.
- when something is thrown away we are putting pressure on the environment's ability to cope - in terms of the additional environmental impacts associated with extracting the new resources, manufacturing and distributing the goods, and in terms of the environmental impacts associated with getting rid of our rubbish.
- when something is thrown away it is lost as possible resource - what is waste to one person may be a possible useful source for another.
Waste can be divided into different types. The most common classification is by their physical, chemical and biological characteristics. One important classification is by their consistency:
- Solid wastes are waste materials that contain less than 70% water. This class includes such materials as household garbage, some industrial wastes, some mining wastes, and oilfield wastes such as drill cuttings;
- Liquid wastes are usually wastewater's that contain less than 1% solids. Such wastes may contain high concentrations of dissolved salts and metals.
- Sludge is a class of waste between liquid and solid. They usually contain between 3% and 25% solids, while the rest of the material is water dissolved materials;
- Gaseous wastes;
- Waste energy.
Another classification is based on the toxic waste influence on the human health – according to this principle wastes are divided into two classes: hazardous and non-hazardous.
The largest waste classification is by the source of generation – for example inert waste, kitchen waste, litter organic waste, medical waste, municipal solid waste, packaging waste, electronic waste, farm waste, food waste, green waste, commercial waste, construction and demolition waste etc.
The most important classification of waste is based on the possibility for recycling and divides waste into recyclable (plastics, paper, glass, metal etc.) and non-recyclable.
Waste management conceptions. There are various conceptions about waste management. Conception of “3Rs” classifys waste management strategies according to their desirability in terms of waste minimization ("
waste hierarchy")
- R1 - reduce - reduce the amount of produced waste;
- R2 - reuse - using things again instead of throwing them away after you have finished with them.
- R3 – recovery (recycle materials or recover energy) - includes recycling, composting and energy recovery.
The last option of the hierarchy is “disposal “- via landfill or landraise.
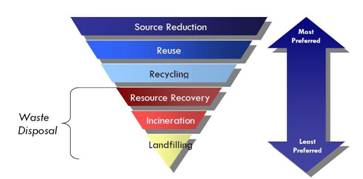
Fig.1. Waste hierarchy
According to this hierarchy the best way and the most desirable option of managing waste is not to produce it (waste prevention) or to reduce the amount of produced waste (fig.1). Then there may be an option to reuse the material. The waste hierarchy specifies the following order of preference for dealing with the wastes: with those towards the top of the list more desirable than those towards the bottom (
disposal).
In most cases a combination of options for managing the different wastes produced at home and at work is needed. The problem with the generated waste today is that more of the rubbish is dealt with towards the bottom end of the hierarchy than the top. The challenge is to change our attitudes and our practices so that much more of generated waste is dealt with by options towards the top of the hierarchy.
Another waste management conceptions are:
-
Extended producer responsibility is a strategy designed to promote the integration of all costs associated with products throughout their life cycle (including end-of-life disposal costs) into the market price of the product. This means that firms which manufacture, import and/or sell products are required to be responsible for the products after their useful life as well as during manufacture.
-
Polluter pays principle - the Polluter Pays Principle is a principle where the polluting party pays for the impact caused to the environment. This generally refers to the requirement for a waste generator to pay for appropriate disposal of the waste.
Waste management alternatives. Different managing alternatives can be applied to waste, depending mainly on its characteristics. Between the most important waste treatments are sorting and recycling (mechanical and chemical), energy recovery (incineration), biodegradation and finally - landfilling.
Sorting and Mechanical recycling. Sorting and mechanical recycling allows the recovery of raw materials, which can be later used to manufacture new products. Sorting could be done by consumers (by collecting waste separately) or at sorting units. Two types of mixed solid waste are accepted at the sorting units:
- Household solid waste: packaging (glass, plastic, aluminium, cardboard and steel) as well as newpapers and magazine etc.;
- Commercial and industrial waste: card, paper, wood, metal, plastic, building site waste etc.
Once the mixed waste arrives at the sorting unit, it takes place an initial pre-sorting and a first phase of mechanical sorting (fig.2). After that, a manual process is applied and material is separated in two different groups: recyclable material and non-recyclable material. The last one is normally sent to incinerating facilities or to landfill.

Fig.2. Waste sorting

Fig.3. Waste baler
Mechanical recycling combines methods for waste treatment without chemical braking. Recyclable material is mechanically treated for size reduction (if necessary) using shredders, cutters, crushers), packed with compactors and balers (fig.3) and sent either to recycling facilities or they are supplied to industry for reuse (for example, card for the paper industry, plastics, metals, glass etc).
Chemical recycling. Waste chemical recycling is based mostly on thermal decomposition of treated materials by breaking of chemical bonds at high temperature.
Pyrolysis and
gasification are thermal processes which break down waste, containing carbon such as paper, petroleumbased wastes (plastics), and organic materials such as food scraps, using high temperatures.
Pyrolysis involves heating waste in the absence of oxygen at temperatures of 400-800°C. The heat alone breaks down complex molecules and the resultant gases are then passed into a combustion chamber where they are burned (in the presence of oxygen) at temperatures around 1250°C.
Gasification involves heating wastes in a low-oxygen atmosphere to produce a gas with a low energy content. This gas can then be burned in a turbine or engine. The resulting product is synthetic gas (
syngas) consisting of mainly carbon monoxide CO and hydrogen H2 (nearly 85%), ad small amounts of carbon dioxide and methane. Syngas has a high calorific value, so it can be used as a fuel to generate electricity or steam, or used as a basic chemical in the petrochemical and refining industries. Other by-products include liquids (mainly water, used for washing the gas clean) and solid residues – ash or char. Typical gasification and pyrolysis processes have the following steps (fig.4):
- Pre-treating the waste - usually involves separating out some of the materials which have no calorific value or are recyclable (glass, grit and metal etc.);
- Heating the remaining waste, mainly organic pulp, to produce gas, oils and char (ash);
- Cleaning the gas to remove some of the particulates, hydrocarbons and soluble matter (scrubbing);
- Application of the cleaned gas to generate electricity or heat.
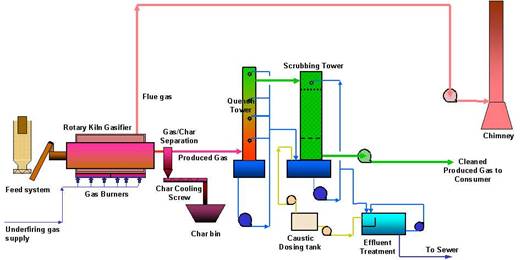
Fig.4. Schematic view of waste pyrolysis process
A good approach to generate energy from waste is the combination of pyrolysis with gasification process as a second stage: the pyrolysis process degrades waste to produce char (or ash), pyrolysis oil and synthetic gas at the first stage; the gasification process then breaks down the hydrocarbons left into syngas using a controlled amount of oxygen.
Energy recovery (Incineration).
Incineration allows obtaining energy and, at the same time, reducing the volume of landfilled waste. The high combustion temperature makes it possible to recover energy from the waste and use it for heating, industrial applications and electricity production. Main technologies for the incineration of waste are:
Mass Burn - This is the simplest and most common form of incineration. Mixed wastes are fed into a hopper and then fall onto a sloping grate which agitates and moves the waste through the combustion chamber. Energy is recovered from the hot combustion gases, which is used to generate electricity;
Fluidised Bed Combustion - Before the waste is incinerated, non-combustible components are removed and the waste shredded to produce coarse refuse derived fuel which has a higher calorific value than the untreated waste. This fuel is fed into a bed made up of a mixture of sand and dolomite mineral. Air is pumped through the base so that the solid waste and minerals resemble a bubbling liquid. This ‘fluidisation’ improves the combustion efficiency, hence reducing pollution and generating more energy per tonne of waste.

Fig.5. Hundertwasser Spittelau Incinerator (Austria,Vienna)
Three different kind of waste are usually used in the incineration facilities: household waste, non-hazardous industrial waste and rejects from sorting plants. In the incineration process, waste collection vehicles dump the waste in vast trenches where it is mixed and transferred to an oven. Burning waste at extremely high temperatures also destroys chemical compounds and disease-causing bacteria. The ash formed in the combustion process is used for beneficial use such as daily cover in landfills and road construction. A modern incineration facility is equipped with air pollution control devices such as electrostatic precipitators, dry and wet scrubbers and/or fabric filters, which are used to remove potentially harmful particulates and gases from the emissions of the unit. State-of-the-art technical decisions of incineration process prevent the environmental pollution and allow incinerators to be situated even in the very central zone of cities without risk for the human health (fig.5).
Biodegradation.
Biodegradation is a process of chemical breakdown of materials by environment. Organic material can be degraded aerobically with oxygen, or anaerobically, without oxygen. Biodegradable matter is generally organic material such as plant and animal matter, substances originating from living organisms, artificial materials that are similar enough to plant and animal matter to be put to use by microorganisms.
 Aerobic biodegradation
Aerobic biodegradation. The process of aerobic biodegradation of organic mater is also known as
composting. Composting is a process that has always existed in its natural state. Today, with modern technologies, this process can be accelerated and monitored efficiently. In general, composting is the process of controlled aerobic decomposition of biodegradable organic matter. During composting, microorganisms break down organic matter into carbon dioxide, water, heat, and compost:
Organic matter +O2 → compost + CO2+H2O+NO3- +SO42- + heat
There are a large number of
biodegradable materials suitable for composting: food and drink industry waste; paper, card, timber and other cellulose-based biodegradable waste; household waste; organic sludge including sewage; agricultural waste. Some waste biodegradable materials should not be used in household compost because they attract unwanted vermin and do not appropriately decompose in the time required: meat, diary products, eggs.
Microorganisms are key to composting. According to the oxygen consuming microorganisms could be divided in aerobic (use oxygen for their metabolism) and anaerobic (they are active in environment without oxygen). The process has three stages: active (thermophilic) biodegradation, cooling and maturation. Temperature is a key parameter determining the success of composting process. During the first stage the temperature reaches 55-65°C, and during the second one - 35 - 45°C. Heat is produced as a by-product of the microbial breakdown of organic material. Temperatures t< 25°C could end of the composting process. Temperatures t > 70°C could kill also bacteria responsible for composting process. Moisture is the second important for the microorganisms metabolism factor – the optimal moisture level is 50 – 60%. Carbon C, nitrogen N, phosphorus P and sulfur S are elements naturally existing in biomass. Carbon-to-nitrogen, carbon-to-phosphorus and carbon-to-sulfur balanced ratios are also important for the process. The value of pH changes during the composting process also – it decreases during the first stage because of CO2 generation, but increases during the second stage up to 8-9 because of ammonia NH3 generation.
According to the process conditions there are two types of aerobic composting:
active (hot) and
passive (cold) composting. The passive composting is more slow than the hot one and is preferred for domestic composting. According to the technical performance composting could be performed as enclosed (home
container composting or industrial
in-vessel composting and composting in exposed piles (home composting in pile and industrial
windrow composting (fig.6).
The final product from the composting process is mature and completely disinfected compost. There are several ways to determine the degree of compost’s stability achieved, based on the determination of oxygen uptake rate, low degree of reheating in curing piles, organic content of the compost, presence of nitrates and the absence of ammonia and starch in the compost. How to decide if the compost is ready for usage? It should look like dark crumbly soil mixed with small pieces of organic material, with brown color and should have a pretty earthy smell.
There are many
benefits from home composting. The ready compost can be used in gardening and agriculture as a soil amendment for erosion control, land/stream reclamation, wetland construction, as landfill cover or as a seed starting medium.

Home composting

Industrial in-vessel composting

Industrial Windrow composting
Fig. 5. Composting techniques
Anaerobic digestion.
Anaerobic digestion is a biological process conducted in the absence of oxygen in which micro-organisms degrade the organic fraction of a feedstock, yielding a product gas (biogas) of principally methane and carbon dioxide and with some trace gases such as H2S, N2, NH4, etc.
In the anaerobic digestion process, the organic portion of the waste is separated to remove plastic, glass and metals and then placed in a sealed reactor. The process is similar to the one that occurs naturally in landfills. Anaerobic digestion is a series of processes in which
microorganisms break down biodegradable material in the absence of oxygen, used for industrial or domestic purposes to manage waste and/or to release energy. There are four key biological and chemical stages of anaerobic digestion (fig.6):
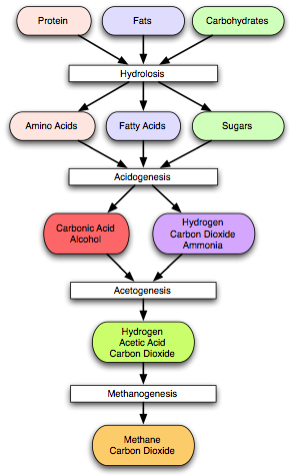
Fig.6. Anaerobic digestion process
- Hydrolysis: the complex organic molecules are broken down into simple sugars, amino acids, and fatty acids by enzymes;
- Acidogenesis (Fermentation): the biological process of acidogenesis is where there is further breakdown of the remaining components by acidogenic (fermentative) bacteria; here volatile fatty acids are created along with ammonia, carbon dioxide and hydrogen sulfide as well as other by-products;
- Acetogenesis: molecules created through the acidogenesis phase are further digested by acetogens to produce largely acetic acid as well as carbon dioxide and hydrogen;
- Methanogenesis: methanogens utilise the intermediate products of the preceding stages and convert them into methane, carbon dioxide and water.
A simplified generic chemical equation for the overall processes outlined above is as follows:
C6H12O6 → 3CO2 + 3CH4
Anaerobic digestion degrades organic matter and makes the resultant residue more stable; this protects the environment from the uncontrolled degradation of the waste. Thus, it reduces the potential for the production of atmospheric methane and leachate. It also reduces the impacts from environmental aspects such as odour, flies and vermin and helps to reduce the plant and animal pathogens that can be spread by wastes. The biogas yielded by the process can be used for various applications. An efficient option is to use the biogas in a combined heat and power (CHP) plant, in which heat and electricity are generated simultaneously
 Landfill
Landfill. Waste that can neither be reused nor receive special processing is stored in landfills. Beforehand, some waste may need to be stabilised to prevent pollution leakage.
A landfill requires high technical standards and must conform to strong safety norms in order to protect the environment and local communities. It is very important taking into account:
- The location for the landfill and the surrounding land
- The design of modular compartments
- The protection of groundwater
- The collection, treatment and elimination of leachates
- The capture, treatment and recovery of biogas
- The construction of a cover, in order to minimise rainwater penetration
Waste is monitored and weighed as it enters the site. Waste with high pollution levels and toxic waste is redirected to a special site. Waste is stored in isolated independent cells and then covered with geological screens in the form of layers of impermeable materials (eg. clay) topped with watertight and drainage systems. By this way all contact between the waste and the natural environment is prevented. Finally, with the view of revegetation it is added a layer of topsoil. Over time, waste, which decomposes produces
landfill gas or biogas, a mixture of carbon dioxide and methane. Biogas is collected and then used in co-generation processes, which produce energy and heat. Apart from biogas, the decomposition of landfilled waste, along with rainwater infiltration, produces a liquid known as "
landfill leachate". Leachates contain heavy metals, salts, nitrogenous compounds and various types of organic matter. Due to its high polluting potential it is needed that this leachate is collected and treated to avoid environment contamination. Modern landfills are well-engineered facilities that are located, designed, operated, and monitored to ensure compliance with European regulations.


 The problem with waste. Waste or rubbish is what people throw away because they no longer need it or want it. In the real Nature the word ”waste” does not exist – everything thrown away by a creature or naturally dead could be of huge importance for the lifecycle of another creature. In difference, almost every human activity generates waste. The fact that people produce waste, and get rid of it, matters for the following reasons:
The problem with waste. Waste or rubbish is what people throw away because they no longer need it or want it. In the real Nature the word ”waste” does not exist – everything thrown away by a creature or naturally dead could be of huge importance for the lifecycle of another creature. In difference, almost every human activity generates waste. The fact that people produce waste, and get rid of it, matters for the following reasons:



 Aerobic biodegradation. The process of aerobic biodegradation of organic mater is also known as composting. Composting is a process that has always existed in its natural state. Today, with modern technologies, this process can be accelerated and monitored efficiently. In general, composting is the process of controlled aerobic decomposition of biodegradable organic matter. During composting, microorganisms break down organic matter into carbon dioxide, water, heat, and compost:
Aerobic biodegradation. The process of aerobic biodegradation of organic mater is also known as composting. Composting is a process that has always existed in its natural state. Today, with modern technologies, this process can be accelerated and monitored efficiently. In general, composting is the process of controlled aerobic decomposition of biodegradable organic matter. During composting, microorganisms break down organic matter into carbon dioxide, water, heat, and compost:




 Landfill. Waste that can neither be reused nor receive special processing is stored in landfills. Beforehand, some waste may need to be stabilised to prevent pollution leakage.
Landfill. Waste that can neither be reused nor receive special processing is stored in landfills. Beforehand, some waste may need to be stabilised to prevent pollution leakage.