

Chemistry is all around us
Copyright 2015
This project has been funded with
support from the European Commission
Educational Packages
Chemistry at Home
Chemical substances in foods
Introduction
Nutrients in foods Foods contain six types of nutrients. Carbohydrates, fats, proteins, vitamins, minerals and trace-elements, and water are considered nutrients. Sufficient quantities of these nutrients are obtained from foods, in order to cover the needs of a living organism. Many times during the treatment of foods we add several chemical substances. Food additives are natural or artificial substances added to food to preserve or enhance its color, flavour, taste and appearance.
Foods contain six types of nutrients. Carbohydrates, fats, proteins, vitamins, minerals and trace-elements, and water are considered nutrients. Sufficient quantities of these nutrients are obtained from foods, in order to cover the needs of a living organism. Many times during the treatment of foods we add several chemical substances. Food additives are natural or artificial substances added to food to preserve or enhance its color, flavour, taste and appearance.
 When you want to bake bread or a cake you have to add a material in order to make it rise. Baking soda and baking powder are such materials. The rising of bread or a cake occurs with the addition of baking powder or baking soda. These substances react with certain ingredients of dough and a gas (carbon dioxide: CO2) is released allowing the bread to rise. Therefore, they are called raising agents.
When you want to bake bread or a cake you have to add a material in order to make it rise. Baking soda and baking powder are such materials. The rising of bread or a cake occurs with the addition of baking powder or baking soda. These substances react with certain ingredients of dough and a gas (carbon dioxide: CO2) is released allowing the bread to rise. Therefore, they are called raising agents.
 Baking powder (a white powder) is a dry material used as a raising agent. It consists of three basic components: baking soda (sodium bicarbonate), tartaric acid, and corn starch. When baking powder is exposed to water, the first two of the above components react to form carbon dioxide gas, producing bubbles and allowing the dough to rise. The label of the commercial product indicates that corn starch is the ingredient with the highest proportion. Why should corn starch exists in such a high proportion since it does not participate in any chemical reaction? There are three reasons why this occurs: 1) Corn starch helps the product remain dry and prevents clotting, 2) Corn starch retains sodium bicarbonate and tartaric acid dry preventing their reaction while stored and 3) Corn starch increases baking powder’s mass and therefore makes measurements easier. It is easier to have to add a spoonful of a powder to a recipe instead of a few grains.
Baking powder (a white powder) is a dry material used as a raising agent. It consists of three basic components: baking soda (sodium bicarbonate), tartaric acid, and corn starch. When baking powder is exposed to water, the first two of the above components react to form carbon dioxide gas, producing bubbles and allowing the dough to rise. The label of the commercial product indicates that corn starch is the ingredient with the highest proportion. Why should corn starch exists in such a high proportion since it does not participate in any chemical reaction? There are three reasons why this occurs: 1) Corn starch helps the product remain dry and prevents clotting, 2) Corn starch retains sodium bicarbonate and tartaric acid dry preventing their reaction while stored and 3) Corn starch increases baking powder’s mass and therefore makes measurements easier. It is easier to have to add a spoonful of a powder to a recipe instead of a few grains.
 Every chemical reaction proceeds with a different rate. The rate of a chemical reaction depends on how quickly the molecules of the reactants react in order to form the molecules of the products. It is known that there are some chemical reactions that are performed with higher rate than others. For example, the reactions that take place in the explosion of a bomb are performed with a high rate, while the transformation of graphite into diamond or the oxidation of iron (rusting) are done very slowly and may take even years to happen.
Every chemical reaction proceeds with a different rate. The rate of a chemical reaction depends on how quickly the molecules of the reactants react in order to form the molecules of the products. It is known that there are some chemical reactions that are performed with higher rate than others. For example, the reactions that take place in the explosion of a bomb are performed with a high rate, while the transformation of graphite into diamond or the oxidation of iron (rusting) are done very slowly and may take even years to happen.
 There are many factors that can affect the rate of a chemical reaction. These factors are: 1) The temperature in which the chemical reaction is performed, the rate of which is generally increased when the temperature increases, 2) The concentration of the reactants. The reaction rate increases as long as the concentration increases. 3) The contact surface of the solids. If the contact surface of a solid is increased the rate of the reaction increases as well. 4) The presence of substances that act as catalysts. These substances always increase the rate of the reaction.
There are many factors that can affect the rate of a chemical reaction. These factors are: 1) The temperature in which the chemical reaction is performed, the rate of which is generally increased when the temperature increases, 2) The concentration of the reactants. The reaction rate increases as long as the concentration increases. 3) The contact surface of the solids. If the contact surface of a solid is increased the rate of the reaction increases as well. 4) The presence of substances that act as catalysts. These substances always increase the rate of the reaction.
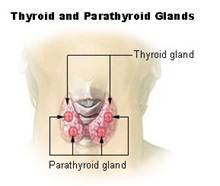 Iodine is a non metal element and is characterized as a micronutrient. Micronutrients are nutrients required by humans throughout life in small quantities in order to orchestrate a whole range of physiological functions, which however cannot be produced by the human body itself.
Iodine is a non metal element and is characterized as a micronutrient. Micronutrients are nutrients required by humans throughout life in small quantities in order to orchestrate a whole range of physiological functions, which however cannot be produced by the human body itself.
 Table salt’s main ingredient is sodium chloride (NaCl), which absorbs the atmosphere’s moisture and tends to clot when it is exposed to air with high humidity. In order to prevent table salt’s clotting we add chemical substances, like silicon dioxide (SiO2) and calcium phosphate (Ca3(PO4)2), which absorb moisture and retain table salt dry.
Table salt’s main ingredient is sodium chloride (NaCl), which absorbs the atmosphere’s moisture and tends to clot when it is exposed to air with high humidity. In order to prevent table salt’s clotting we add chemical substances, like silicon dioxide (SiO2) and calcium phosphate (Ca3(PO4)2), which absorb moisture and retain table salt dry.