Chemical Analysis of Historical Materials and Cultural Heritage Objects
Activities
Jewels of Amber from the Baltic Sea
Ambers are the fossilized resins from prehistoric trees. Amber has been appreciated for its colour and natural beauty and therefore used since the ancient time for making jewelry. Amber can be classified in terms of place of its origin, as called Baltic amber comes from the region around the Baltic Sea. The origin of this type of amber can be identified by infrared spectroscopy in which case we identify on the spectrum so called "Baltic shoulder", that is hidden absorption band with a peak at wavenumber 1150-1210 cm-1 (Fig. 3). If this shoulder in the spectrum is missing, amber comes from another region of the world. This information helps the archaeologists to complete the survey on ancient
amber trade routes.
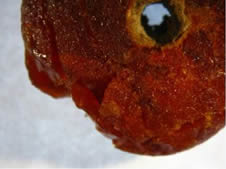
Fig. 1 Photo of an amber bead of unknown origin, that was found in the tomb in Bohemia region.

Fig. 2 Photo of Baltic amber bead, which was used as the standard.
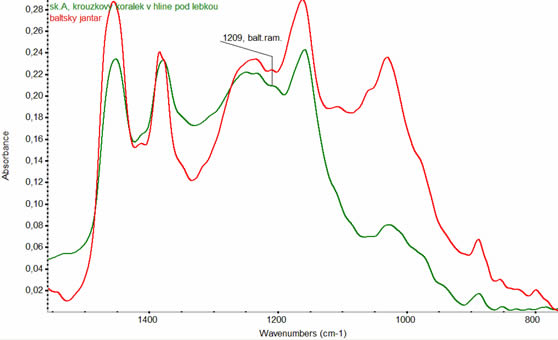
Fig. 3 FT-IR spectra (Green curve - a comparative spectrum of the Baltic amber with a characteristic "Baltic shoulder" i.e. absorption band with a peak at wavenumber 1150 - 1210 cm-1. The red curve - spectrum measured on a sample of the bead ring found in a tomb under the skull. In both spectra we identify the "Baltic shoulder", it proves that the amber bead found in the thumb is made of Baltic amber. We can conclude that at the place of finding our ancestors traded with regions around the Baltic Sea.)
Reconstruction of the Gothic Charles Bridge in Prague
The Charles Bridge, one of the gothic jewels of Prague architecture, is currently undergoing extensive reconstruction, which is under the scrutiny of the general public. Causes of degradation and quality of the reconstruction work are broadly discussed by professionals, and many scientific and popular articles have been published in press and other media.
The facing masonry of the Gothic Charles Bridge in Prague (Czech Republic) has been largely
altered during nineteenth and twentieth century repairs, due to extensive replacements of weathered sandstone ashlars blocks. Natural stone varieties used during these replacements show different responses to a variety of weathering processes which lead to their rapid decay. The decay of the newer stone has been accelerated by use of
Portland cement paste makes an almost of the binding and fill material, instead of the original hydraulic lime-based and “cocciopesto” type mortars. The hardened Portland cement created an impermeable barrier, with permeability three to four orders of magnitude lower, compared to the original mortars and natural stone. The low permeability of this new alien material resulted in the accumulation of water-soluble salts (specifically nitrates, but also sulphates and chlorides) in the facing masonry ashlars, with their crystallization coming in the form of both sub- and efflorescence, as well as the development of various decay forms (blistering, granular disintegration and/or scaling and flaking).
Another problem is the presence of polymers, which were used to treat facing brickwork in the 20th century. Under the impermeable black crust, the merry life of microorganisms is thriving that caused the mass of stone becomes breakable and friable.
How would you proceed to identify the causes of bridge masonry degradation? What analytical method would you suggest? What you find from the measured results?
The first step is the sampling and making the microscopy analysis of the sample cross section in visible light (Fig. 4) and UV light (Fig. 5).
In the UV light we can observe a typical strong pink fluorescence of micro-organisms.
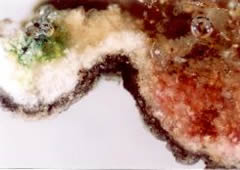
Fig. 4 Microscopic photo of the stone hand railing in visible light.

Fig. 5 Microscopic photo of the stone hand railing in UV light with pink fluorescence.
The second step is to analyze the samples by FT-IR spectroscopy. Evidence of the presence of microorganisms is confirmed by the presence of the pectinase enzyme (metabolic product of microorganisms), which was identified in the measured spectra (Fig. 6).
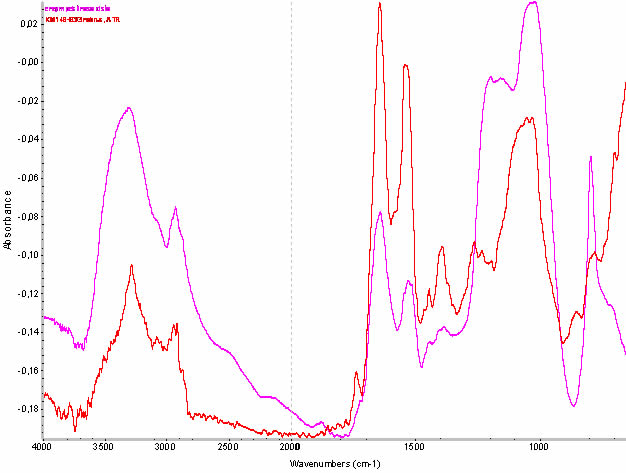
Fig. 6 The red curve - FT-IR spectrum measured on a sample of masonry. Purple curve - a comparative spectrum of pectinase enzyme, produced by micro-organisms. The characteristic absorption band of protein (pectinase enzyme) is at the spectrum wavenumber 1640 and 1520 cm-1.







