Chemical Analysis of Historical Materials and Cultural Heritage Objects
Introduction
Chemistry and Hidden Secrets of Historical Objects
Every person is looking for explanations for current events in the past, learns from the past, searches for roots and the information that would bring new ideas into his or her life. Any object, which withdraws from the depths of the past, tells the story of its origin, of technology that was used to make it, of habits and lives of people who had used it, of its way over time.
Where once one man was enough - an artist and technologist at the same time, now we need to put together several different fields of human activity. Artists and scientists (such as painters, sculptors, craftsmen, historians, archivists, linguists, physicists, chemists and biologists) are forced to work closely together so that they examine, document, conserve, restore and then finally exhibit objects in its full beauty. Then, monuments and art works can tell their stories for delight and education for all of us. Chemistry thus becomes one little stone of the mosaic of patient work. The work that only from time to time brings excitement and moments of unexpected discoveries.
This collaboration of artists and scientists have its rules, given not only by laws but also by the unwritten ones, which allows to use ingenuity and imagination of artists and combines it with the sobriety of scientific procedures that prevents publishing of discoveries and hypotheses without their verification.
Exploration and preservation of historic and artistic heritage is a field broadly observed and pursued with the photogenic and attractive potential of fame. Therefore, it is necessary to evaluate very carefully and critically all information received and revalidate it from time to time using newly discovered methods and procedures of modern science. The invention of humanity is high, desire for ownership boundless and counterfeiters got busy even during the ancient civilizations. One of the most famous counterfeiters operating in the 20th century was the Dutchman
Han van Meegeren. The boom of chemistry and its new methods of analysis, i.e. spectroscopy, had its share on his disclosure.
Many world-famous sights have their mysteries; we should remember for example
The Shroud of Turin. Suspicion of falsification have not missed also
The Snake Goddess originally considered as an evidence of the worship of matriarchal divinity of Minoan culture of Crete, now stored in
Boston Museum.
Every day forgotten or previously unknown works of art or lost artifacts are discovered, every day it is necessary to provide care of historical monuments and museum collections. In these cases, the chemistry is one of many fields, but the field that provides irreplaceable information about materials and technologies of the work. Without this knowledge, it is impossible to ensure compatibility of old and new materials used for conservation and restoration of the work.

Fig. 1
Also in the Czech Republic there are many historical monuments, for which the scientific research including the chemical one ensures the best conditions for their protection and preservation for future generations. And so now we can follow almost detective search for the fate of
The Reliquary of St. Maur an important Romanesque monument displayed in the
Bečov castle in Western Bohemia.
We can marvel at the skills of our ancestors when looking at the
Charles Bridge in Prague.
Czech Crown Jewels reluctantly issued some of their secrets during the mineralogical survey, but other unknown is still necessary to find and hopefully also the chemistry will play its role.
Come with us on the path of revealing history and art by non-traditional means and methods of science, enter the world of chemistry, and in the future you can align with many professionals from research institutes, universities, museums, galleries and foundations that reveal the secrets of the past.
Scientific Research of Landmarks, Monuments and Historical Artwork - Analysis Planning
For our main purpose, to rescue and restore objects of historical value, we need to focus on comprehensive research of our cultural heritage. Such research is truly interdisciplinary and requires cooperation of chemists, restorers, artists, conservationists, owners and historians. Chemical analysis of materials used for creating all sorts of subjects is one of the most important parts of the research.
There are several characteristics of the object we need to know to be able to carry out the chemical analysis – for example we have to look into the history of the object, we should check its changes in time, place of storage and material in which it was stored, research production technology, previous conservations etc.
The analysis planning process also respects reasons and objectives of the research. We also have to look into chemical mechanisms and interactions especially on the interface between original and modern materials. Knowledge of degradation processes is also essential.
Before extracting the testing sample for our analysis we should raise following questions:
- How was the object made?
- Where was it made (location)?
- What is the objects history?
- What is the purpose of our analysis?
- What other information we need?
- Are all the parts of the object authentic?
- Why was it made?
- To whom it was intended?
We are able to give some answers thanks to the chemical and physical analytical methods and we can base some answers on consultations with historians. However, scientific research is not able to answer the last two questions; it can only give some useful hints that have to be interpreted by the interdisciplinary team.
Principles of Analytical Methods Using Electromagnetic Radiation
Most of the contemporary analytical methods, that are used to research our cultural heritage, should be very gentle and non-destructive. One of the most suitable methods used is
spectroscopy, which is based on the interaction between matter and radiated energy.
Using spectroscopy we can inspect not only the microworld, but also cosmic distances, reveal distant past or view the human body painlessly. Spectroscopy is also used in industry and agriculture, in some cases even criminology. Depth insight into the physical world depends on energy and wavelength of radiation that we use to study.
The light that we are able to perceive with our eyes is an electromagnetic radiation of a wavelength of 400-750
nm. When we admire the beauty of the country with our eyes, we actually evaluate data, that reach our eyes in the form of an electromagnetic radiation, and we are a sort of „marching spectrometer“ that uses the sun as a source of radiation, our eyes as a detector, and finally use our brain as an analytical extension.
Similarly as in the nature, in our
spectrometer we must have a source of the radiation, some optical element to disperse the radiation according to its wavelengths. Then we need detector to identify amount and wavelength of radiation that went through the sample or that was reflected or scattered. We also examine, whether the wavelength was changed by this process. Basically, this is the core of
spectroscopy.
To understand this better, we will try to sort and categorize all sorts of radiation.
Electromagnetic radiation carries a certain amount of energy, in the same time it is also surging. It has waves of different frequencies and frequency is associated with the concept of
wavelength.
Fig. 2
The higher frequency radiation, the shorter the wavelength and higher energy. The greater the amount of energy the electromagnetic radiation carries, the more dangerous it is for the living organism.
We have radiation of one wavelength, it is called monochromatic. If the radiation carries with it a number and sometimes an infinite number of wavelengths, the radiation is polychromatic. Typical polychromatic radiation is sunlight.
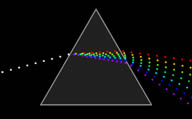
Rays of light break when move from one environment to another. For example, when light falls obliquely on a transparent material like glass or water. Different materials slow down light differently, fracture occurs always under a different angle. In addition to light it applies to all
electromagnetic radiation.
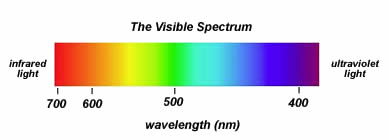
Fig. 4 The Visible Spectrum.
Electromagnetic radiation can be divided according to wavelength of radiation
- electron radiation, the radiation of elementary particle stream of electrons, the wavelength is very small, in the order of picometer, but it also entails huge amounts of energy that could even kill us,
- ultraviolet (UV), whose wavelength is slightly longer, but still in the tens to hundreds of nanometers and it is also dangerous for us. It radiates the sun, but the human eye can not perceive
- visible light, or let us call it the light that it lets us live, is the wavelength of 400-750 nm
- Infrared (IR) belongs among the long-wavelength radiation with a wavelength (760 nm to 1 mm). It is greater than visible light, but smaller than the microwave radiation. Sources of this type of radiation are heated objects, fire, and of course the sun. In addition to red visible light it also carries thermal radiation, but we cannot see it, because our eye is not adapted to it.
- Microwave radiation has long waves, which allows us for example to examine the parameters of the universe. We also use it in the microwave oven
- The Longest waves are radio waves
The wavelengths used in spectroscopy are very tiny, they are used in order of micrometers, which is a millionth of a meter or even less, which is a
nanometer. And these small sizes actually bring us information that we can very well used in analytical chemistry and also in real life.
Radiation can also be divided by the physical phenomena observed, so we have emission radiation, absorption radiation and fluorescence radiation, and all these phenomena can also be used analytically. Do you know what emission of radiation means?
In the microworld, there is everything in constant motion and even we, as we sit still, are in constant move inside. Electrons in us circulate their orbits around the nuclei, atoms
vibrate chemical bond of molecules, and all this movement, when we shine on it or when we warms it up, even fastens. If we add some amount of energy into the measured system, "we lit the infrared light on it", we excite the system and energy charge increases. However, in nature everything tends to be balanced, so this amount of energy absorbed by the sample initially at its excitation wants to go back and out. Therefore it is emitted from the sample and that is what we call emission of radiation that can be used analytically, as well as absorption. During the excitation, the molecule must absorb certain amount of energy. When we measure the difference in radiation intensity we obtain information on chemical structure of the sample.
So can we say that spectroscopy is the gift of physics to the chemists; based on the knowledge of spectroscopy, chemists can study radiation emitted or absorbed by the examined samples or objects and identify an unknown substance from the
spectrum emitted.
Methods Used by the Chemical Analyst
In our survey of historical and artistic subjects we usually begin with
imaging methods, which allow to capture photographic documentation in a different light - visible, ultraviolet, red and infrared; we can image the x-photographs as well as we show the human body. Further we make pictures with different magnification - technology that refine our vision so that we can explore even small details of the subject. For this, we use optical
microscopes, fluorescence microscopy and electron microscopy that lead us to the image analysis. Based on the shape and size of fine fractions we can than infer the composition of the monument.

Fig. 5 Cross section of plaster strata in visible light.

Fig. 6 Cross section of plaster strata in UV light; we can observe the typical fluorescence of shellac. Orange layer represents the fluorescence of shellac paint; red fluorescence is a mixture of shellac and plaster.
If you need to know what chemicals are present in the examined subject, we have to use another type of analytical method. In this case we will reveal secrets of the chemical structure using the infrared light that can "poke its nose” everywhere and measure without the risk of damage of examined sample. This method is especially suitable for analysis of organic compounds, because
infrared spectroscopy allows us to observe vibration bonds in molecules.
This method allows us to get the information from a relatively small sample, and if it fails, it can provide guidance on how to analyze the sample further and how to get additional amount of information that can then be used by restorers.
The principle of this method is the
absorption of infrared radiation passing through the sample, which involves changes in rotational and vibrational states of molecules in response to changes in dipole moment of molecules.
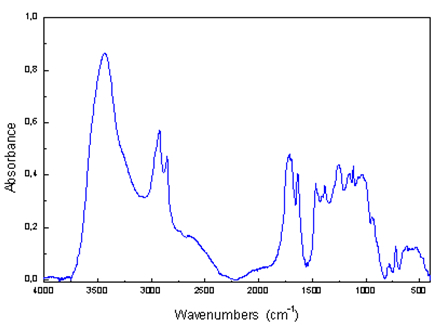
Analytical output is the infrared spectrum, which is a graphic display of the functional dependence of energy, usually expressed as a percentage of transmittance (T) or absorbance units (A) on the wavelength of incident radiation, respectively, the wavenumber, which is the inverse of
wavelength. The absorption bands having peaks at an interval of wave numbers 4000 - 1500 cm-1 are suitable for the identification of functional groups of organic substances (e.g.,-OH, C = O, NH, CH3, etc.). Bands of 1500 - 400 cm-1 are called "fingerprint region”. Using the "Search programs" and digital
libraries of infrared spectra it is possible to identify an unknown analyzed substance by comparing the spectra.
To measure spectrum of the investigated sample we need a laboratory instruments called a spectrometer (link: http://en.wikipedia.org/wiki/Spectrometer) or spectroscope, which process the radiation into spectra thanks to the endowment of mechanics, mathematicians and sophisticated computer programs. From the spectra, we can deduce chemical composition of the investigated objects.
A common laboratory instrument that uses technique of infrared spectroscopy is a Fourier transform infrared (FTIR)
spectrometer (Fig. 8).
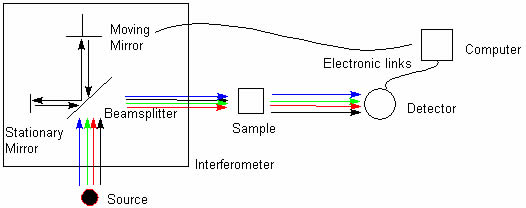
Fig. 8 Scheme of Fourier transform infrared spectrometer (FTIR).
So also this is chemistry, knowledge of which can be used not only for the analysis of art and historical materials, but can also serve in other areas of civic life, in industry, agriculture, health care. Each of us is essentially a chemical reactor and every one of our idea is supported by specific chemical reaction.
Do not be afraid of chemistry, you can come to study for example to ICT Prague. With chemistry, you can experience exciting moments when exploring new worlds. Visit the homepage of the
Department of Chemical Technology of Monument Conservation, or watch the videos from
“Open Doors at ICT Prague”,
laboratory of ceramics and
laboratory of glass.