Chemistry and Metal Objects of Cultural Heritage
Introduction
Chemistry and Metal Objects of Cultural Heritage
What come first in your mind when we say corrosion? You probably remember the reddish rust covering of the old iron objects. But the truth is, that
corrosion affects nearly all materials. It's a very complex problem caused by many factors, as will be explained in following text and as will be demonstrated on concrete examples of historical metal statues.
Most of metals perform, according to the environment, three types of corrosion behavior. The state, when metal does not corrode in the environment is called immunity. A typical example of a metal that is resistant to
atmospheric corrosion due to its immunity is
gold. The rust, that is hydrated oxides of iron, as we mentioned in the introduction of this text, arises because
iron is during atmospheric exposure in state of activity. This means that metal surface corrodes, formed oxides fall off from the surface and discovered “alive” pure metal surface is again oxidized. If the object is not treated in time, the corrosion process ends up by total corrosion of the object, which in fact disappears. The last possible state of the metal is called passivity. In this case, the surface of the metal corrodes as well. The resulting compounds, however, forms a protective layer of corroded material which is the reason of great corrosion resistance such as titanium, aluminum and stainless steel. If the resulting layer is changing the face of the metal surface, it is called a patina. It is a protective layer of corrosion products on the surface of metal object which protects the objects from further oxidation. Patina gives the appearance of historical monuments and it is often created artificially. One example of metal covered by atmospheric exposure by patina is
copper, red metal, first produced in pure form by reduction of ores, approximately 3500 BC. Copper surface, as we all know form roofs of churches and cathedrals, is covered by green
verdigris brochantite, Cu4(OH)6SO4 – hexahydroxo tetra copper sulfate (Fig. 1).
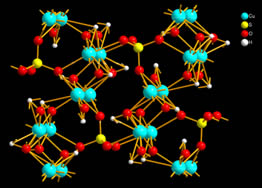
Fig. 1 Brochantite - hexahydroxo tetra copper sulfate.
Currently the most common construction materials are metallic
alloys of iron followed by alloys based on
aluminium.
Since ancient times, copper and its alloys, bronze and brass, were widely used
bronze and
brass. Sculptures and other objects have been also often covered by layer of
precious metal. The most common practice was
gilding, for two main reasons. The first was already mentioned, excellent corrosion resistance of gold, the second reason was to ensure the object looked like made of pure gold.
Let us move now to specific examples, these are the two significant metal sculptures. In this case, the obvious task of renovation is to repair all damaged places, preventive anticorrosion treatment of individual parts, and if it is possible to take such actions, anticorrosion and mechanical, to prevent further destruction of monuments.
For historical monuments with other than purely decorative function, the situation is more complicated. There is a dilemma if the object should be only
preserved or whether functional value has to be returned at the cost of a partial loss of authenticity. In these controversial cases the crucial issue is a request of the object´s owner.
For example renovation of
The Most Holy Trinity Column (Fig. 2) in
Olomouc that represents baroque monument to its importance beyond the border of Moravia and the Czech Republic. It is declared by UNESCO as a part of cultural heritage. Metallic sculptures from
The Most Holy Trinity Column were made from beaten copper sheets. The visible parts of sculptures surface were gilded in fire. The sculptures were provided with internal armature from
wrought iron strips. Wrought iron was also used for hammer forging of anchoring and supporting elements, fixing the sculptures to the column.

Fig. 2 Metallic sculptures on the top of the Most Holy Trinity Column.
The most serious corrosion damage was observed on the wrought iron supporting elements of Archangel Michael statue and on the sun symbol of Holy Spirit, inclusive strips and clamps of central supporting beam of the cross, which was built into the column capital and embedded by lead (Fig. 3).
By corrosion were heavily attacked parts which faced up, due to construction, permanent moisture, trapping dirty and impurities or the corrosion products of copper flowed down to them. Great importance to the corrosion of ferrous parts was the emergence of of the iron-copper
galvanic cell in place of two different metals connection (Fig. 4).

Fig. 3 Massive layer of rust on the central supporting beam.

Fig. 4 Damage of gilded copper skin caused by pressure of voluminous corrosion products from heavily corroded wrought iron framework.
The damaged parts produced from wrought iron were replaced by parts made from
Fe-Cr-Ni austenitic stainless steel. There is a question whether it is ethic to replace historical material with the modern one. In this case only parts of internal supporting construction were replaced, so the appearance of the monument was not affected. Moreover, using of wrought iron for mechanically stressed parts was the only materials option at the time when the monument was made (1716-1753). In terms of current capabilities, the use of inappropriate material for supporting structure is one of the main reasons why the statue should be restored again.
Wrought iron screw bolts, which are in direct contact with gilded copper skin, were replaced with copper bolts. It was solution of the problem caused by the iron - copper galvanic cell and also for pollution of external surface of sculpture by corrosion product of iron. All parts of supporting construction system of the statue were treated with zinc-rich epoxy ester resin primer coating. Bottom part of sculptures copper skin was fitted with drainage holes to reduce a retention of rainwater.
Gilded surface of sculptures was covered by massive crust of copper corrosion products (Fig. 5) that needed to be removed. For this purpose, the combination of chemical dissolution and mechanical cleaning was used. After application of this procedure, it was found out that the original gilding has survived on surprisingly huge area (Fig. 6).

Fig. 5 Head of Christ with massive layer of copper corrosion products.

Fig. 6 Head of Christ after removing of crusts of corrosion products.
Layer of gold produced by fire gilding is characterized by relatively high thickness and high resistance. On the other hand, the layer contains numerous pores. When water gets into the pores, corrosion under of gilded layer takes place (Fig. 7).
Microstructure photographs of the sample cross-section show that the golden layer is in contact only with copper corrosion products at some points. It results to lower adhesion of the gold layer at the points were the bottom layer is corroded. For the partial restoration and retouching of damaged gold plating leaf gilding was used, but most frequently was used acrylate lacquer filled with gold powder. For restoration of special parts, ”up to date” modification of original technology of fire gilding was developed.
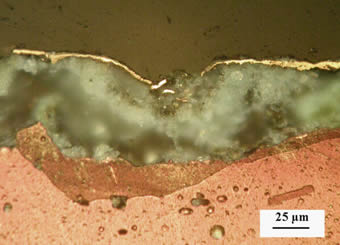
Fig. 7 Microphotograph of damaged gilding on layer of copper corrosion products.
Another example of metal monument that was renovated in cooperation with ICT Prague is the equestrian statue of Bohemian King George of Poděbrady (Fig. 8) that is located on the square of the city
Poděbrady. The statue was made of beaten copper, mounted on the wrought iron framework. Emergency state of the statue, after approximately 100 years of atmospheric exposure, led to the need for total reconstruction of this monument.

Fig. 8 The equestrian statue of King George of Poděbrady.
The main cause of the emergency situation was again inappropriate use and subsequently heavy corrosion of wrought iron framework of the statue (Fig. 9).

Fig. 9 Corrosion damage of iron framework at connection points of two cast iron carrying flanges.
The corrosion damage was accompanied with formation of mechanical cracks on the cast iron parts of the framework and in joints of copper skin (also visible on Fig. 9) The cracks formation was the result of voluminous corrosion products as well as freezing rain water in the cavities of the statue.
For overall restoring of this monument were done several anticorrosion treatments. Damaged parts of the iron framework were replaced with austenitic Cr-Ni stainless steel. Original wrought iron parts were treated with zinc-rich moisture curing polyurethane primer and overcoat with one component polyurethane-tar combination. Additional barrier made of
PTFE tape were installed to eliminate ionic path in the galvanic cell iron armature-copper skin after paint degradation. Drainage holes were fitted in copper skin to drain off rainwater from the inside of the statue. Also proper method of patina coating for the final copper skin surface treatment was selected and tested. All parts of the statue skin were left dismountable that allows regular maintenance of the inside space of the statue.
The state of the monument after executing of all above mentioned treatments is documented in Fig. 10. On the Fig. 9 and Fig. 10 the same detail of the statue before and after renovation is documented.

Fig. 10 Documentation of anticorrosion treatments (carrying flanges from austenitic stainless steel fitted with drainage holes, insulating PTFE tape, protective paint).
After all performed preservation arrangements and anti-corrosion treatments can be assumed that the lifetime of the statue will be extended for hundreds of years.











