Environmental chemistry, chemistry in environment and the human role
Step 2 - Activities
DETERMINING SOIL ACIDITY (pH)
Active reaction of soil. Soil acidity. One of the most important indicators characterizing soil fertility is their active reaction (рН). Soil reaction (soil acidity or alkalization) depends on the substances dissolved in soil moisture and their interaction with hard soil particles It is determined by the ratio between the concentration of hydrogen and hydroxyl ions in the soil solution. A quantitative expression of this ration is the hydrogen indicator pH which in itself is a negative decimal logarithm of hydrogen ions concentration H+ in the medium:
pH = -lg [H+]
The values of pH vary from 0 to 14 ; with pH < 7 the medium is acid whereas with pH > 7 it is alkaline and with pH = 7 it is neutral.
Soils may be distributed over sever groups depending on their active reaction:
- Very acid with рН less than 5;
- Acid with рН 5-6;
- Slightly acid with рН 6-7;
- Neutral with рН 7;
- Slightly alkaline with рН 7-7,5;
- Low alkaline with рН 7,5-8;
- Alkaline with рН greater than 8.
Soil reaction directly impacts the mobility and assimilation of nutrient and non-nutrient elements henceforth it impacts plants growth and development.
Soil acidity also is increased by the large amount of nitrogen based fertilizers used in the form of NH4NO3, CO(NH2)2, (NH4)2SO4. Low humus content in soils allows for the rapid raising of their acidity. Soils also become acid due to contamination with pollutants such as sulphur and nitrogen compounds, acid rains, waste waters from mines etc. Increased acidity of soil causes calcium carbonates to dissolve and be washed away which is highly destructive for the soil structure and deteriorates its physical properties.
pH can be determined by two basic methods:
- calorimetric (by means of indicators);
- potentiometric (via рН-meter).
Calorimeters or pH-meters are used provided that accurate quantitative measurements are required (fig.1). Calorimetric method is applied whenever a quality evaluation is needed during quick tests. This method is based on the color change of a certain type of indicator( paper or liquid type) (fig.2) within the investigated medium and the consequent comparison with a color code range which is typical for this indicator.

fig.1.Determining pH by means of a pH meter
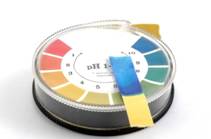
Fig.2. Evaluation of the medium character by means of indicator
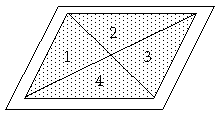
Fig. 3.Taking average soil sample
Determining soil active reaction by way of experiment.
Required materials and tools: 150 – 200 g of soils; 0,5 l distilled water; filtering paper 20x20 cm; 2 glasses: – one is 400 - 500 ml, the other;:100 – 150 ml; funnel and paper indicator.
Step 1: Preparation of soil sample.
Soil in the amount of 100 g is needed for the experiment. Clear the sample from leaves, remnants of branches , pebbles or other larger admixtures. Spread a blanket of soil with thickness of about 1 cm and allow it to dry at ambient temperature. Grind the dried soil sample to remove larger aggregates.
Step 2: preparation of soil extract.
Soil extract is prepared from the dried soil, distilled water or brine of neutral reaction with concentration 1М (for example 1М solution of KCl).
Spread soil over a sheet of paper in the shape of a rectangular layer with thickness of 1 cm approximately Divide the layer by diagonals in four parts 1,2,3 and 4 (fig.3).Take a teaspoonful from sectors 1 and 3. Scramble the rest of the soil and repeat the procedure this time taking a teaspoonful of soil from sectors 2 and 4. Repeat again until you collect sufficient quantity of soil sample needed for the measurement. This way of proceeding guarantees objective results.

Fig. 4

Fig. 5
Weigh 50 g from the obtained soil sample and put in the 400ml glass. Pour 250ml distilled water and stir intensively for about 5 minutes and allow it to stay overnight.
Fold filtering paper along the diagonal and cut it according funnel’s dimensions.Fix the funnel on a post and put a glass of 150ml underneath. Lay the cut out filter and scarcely wet with distilled water. Carefully transfer the upper layer of liquid( soil extract) from the glass containing the soil sample on the filter making sure the liquid does not fall on its upper edge. Collect 50 – 75 ml filtrate in the glass ; this quantity is sufficient to determine soil acidity.

Fig. 6

Fig. 7
Step 3: determining pH and soil characteristics.
Use paper indicator with relevant color code range; each color corresponding to a definite value of pH. Dip a piece of indicator paper in the soil extract for about 1-2 seconds.Then compare the color with the color code range of the indicator. Mark the value corresponding to the obtained color for pH.

Fig. 8

Fig. 9
Now determine the character( active reaction) of the investigated soil sample by using Table 1.
Table 1. Soil classification depending on pH value.
| N |
Value of pH |
Evaluation of reaction |
| 1 |
< 3,0 |
Extremely acid |
| 2 |
3,0 – 3,9 |
Super acid |
| 3 |
4,0 – 4,4 |
Too acid |
| 4 |
4,5 – 4,9 |
Very acid |
| 5 |
5,0 – 5,5 |
medium acid |
| 6 |
5,6 – 5,9 |
Slightly acid |
| 7 |
6,0 – 7,0 |
Slightly acid close to neutral |
| 8 |
7,1 – 7,5 |
Very slightly alkaline |
| 9 |
7,6 – 8,0 (8,2) |
Slightly alkaline |
| 10 |
8,2 – 8,6 |
Medium alkaline |
| 11 |
8,7 – 9,0 |
Alkaline |
| 12 |
9,1 – 10 |
Very alkaline |
| 13 |
> 10 |
Super alkaline |










