Chemistry and energy of the future
Step 2 - Activities
Making “lemon” battery
A lime battery is a kind of galvanic cell. The latter is a chemical source of electric energy in which electric current is produced as a result of chemical reaction. In order to obtain voltage at the lime battery terminals we should be first aware of the chemical processes that occur on the electrodes. The zinc electrode functions as anode. It releases two electrons and the process of reduction takes place. The copper electrode receives two electrons and the process oxidization takes place.
- Anode (А): Zn - 2 e- → Zn 2+ (Electrode potential – Е = - 0,76V)
- Cathode (К): Сu+2e- → Cu 2+ (Electrode potential – Е = 0,34V )
Resultant electric voltage is the difference in the electrode potential of K and A i.e. EDN term.= 0,34-(-0.76) = 1,1V.
A multi-measuring set is necessary to measure resultant electric voltage. The copper electrode is connected to the positive input and the zinc electrode is connected to the negative input.
1.1. Necessary materials(fig.1)
- Two lemons;
- Pocket knife;
- Copper and zinc plates connected with wires;
- Leds or small bulbs of 2V.
1.2. Apparatuses
A multi-measuring set is needed to measure the voltage obtained
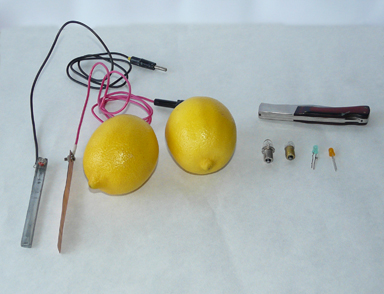
fig. 1. Needed materials
1.3. Description of operations:
Step 1: Use the pocket knife to cut two slots at either end of the lemon and insert both electrodes there (fig. 2).
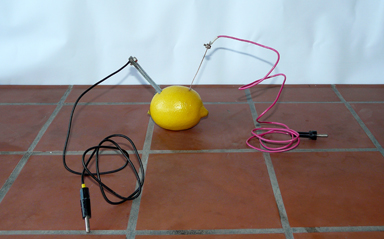
fig. 2
Step 2: Resultant voltage is measured by the measuring set which is set in the range of constant voltage measuring. Copper electrode is connected to the positive input whereas zinc electrode is connected to the negative input of the measuring set (fig. 3). The measuring set display reads the value of the resultant voltage which is about 0.92 V.

fig. 3
Step 3: However, the voltage obtained in this way is not sufficient to demonstrate lemon battery. To obtain adequate electric voltage a second lemon with inserted electrodes is connected to the first one in series; “in series” means that the copper electrode of the first lemon is connected with the zinc electrode of the other lemon. Then electric voltage is measured between the zinc electrode of the first lemon and the copper electrode of the second lemon; its value being about 1.9 V. (fig. 4). In this way the voltage is two times higher and could supply a LED or a small bulb.

fig. 4
Fig. 5 presents the difference between OFF and ON positions of an orange light emitting diode.

Fig. 5
Fig. 6 presents the difference between OFF and ON positions of a green light emitting diode.

fig. 6
To intensify light emission it is possible to use 6 lemons connected in three pairs after which each pair is connected in series as described above. However, to make this task feasible, six zinc and six copper electrodes are needed which may be difficult to provide.
At home it is possible to substitute zinc and copper electrodes with a small coin and nail.









